

Clinical results with SUNOSI
SUNOSI was studied in a clinical trial that included 459 patients with excessive daytime sleepiness (EDS) due to obstructive sleep apnea (O.S.A.).Results measured at 12 weeks with SUNOSI 150 mg showed:
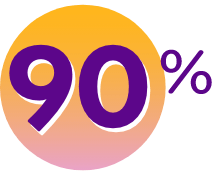
OF PEOPLE REPORTED FEELING BETTER
(compared to 49% with placebo)

INCREASE IN
MINUTES AWAKE*
(compared to 2% with placebo)
What does this mean?
In the study, people taking SUNOSI reported changes at 12 weeks in scores associated with:
THEIR ABILITY TO CARRY OUT DAILY ACTIVITIES
WORK PERFORMANCE
OVERALL WELL-BEING
These are additional analyses that are based on the findings from the main study. These results may have been due to causes other than SUNOSI, and your experience may differ.
Multiple clinical studies also tested the safety of SUNOSI
These studies included over 900 people with EDS due to O.S.A. or narcolepsy.The most common side effects were:
- headache
- nausea
- decreased appetite
- anxiety
- insomnia
- headache
- decreased appetite
- insomnia
- nausea
- anxiety
SUNOSI may cause serious side effects, including increased blood pressure and heart rate as well as psychiatric symptoms like anxiety, problems sleeping, irritability, and agitation.
Remember to report any side effects from prescription medications to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Learn more about the safety of SUNOSI.

Do not take SUNOSI if you are taking, or have stopped taking within the past 14 days, a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
Before taking SUNOSI, tell your doctor about all of your medical conditions, including if you:
- have heart problems, high blood pressure, kidney problems, diabetes, or high cholesterol.
- have had a heart attack or a stroke.
- have a history of mental health problems (including psychosis and bipolar disorders), or of drug or alcohol abuse or addiction.
- are pregnant or planning to become pregnant. It is not known if SUNOSI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SUNOSI passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take SUNOSI.
- SUNOSI does not treat the underlying cause of O.S.A. and SUNOSI does not take the place of any device prescribed for O.S.A., such as a continuous positive airway pressure (CPAP) machine. It is important that you continue to use these treatments as prescribed by your healthcare provider.
Do not take SUNOSI if you are taking, or have stopped taking within the past 14 days, a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
Before taking SUNOSI, tell your doctor about all of your medical conditions, including if you:- have heart problems, high blood pressure, kidney problems, diabetes, or high cholesterol.
- have had a heart attack or a stroke.
- have a history of mental health problems (including psychosis and bipolar disorders), or of drug or alcohol abuse or addiction.
- are pregnant or planning to become pregnant. It is not known if SUNOSI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. SUNOSI passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take SUNOSI.
The most common side effects of SUNOSI include:
- •headache
- •nausea
- •decreased appetite
- •anxiety
- •problems sleeping
SUN CON ISI 06/2023
Please see Medication Guide.
- SUNOSI does not treat the underlying cause of O.S.A. and SUNOSI does not take the place of any device prescribed for O.S.A., such as a continuous positive airway pressure (CPAP) machine. It is important that you continue to use these treatments as prescribed by your healthcare provider.
Do not take SUNOSI if you are taking, or have stopped taking within the past 14 days, a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
Before taking SUNOSI, tell your doctor about all of your medical conditions, including if you:
- have heart problems, high blood pressure, kidney problems, diabetes, or high cholesterol.
- have had a heart attack or a stroke.
- have a history of mental health problems (including psychosis and bipolar disorders), or of drug or alcohol abuse or addiction.
- are pregnant or planning to become pregnant. It is not known if SUNOSI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SUNOSI passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take SUNOSI.





